Molecular Inorganic Chemistry and Catalysis
advancing low-valent main-group chemistry for sustainable synthesis, catalysis, and materials
RESEARCH AREAS
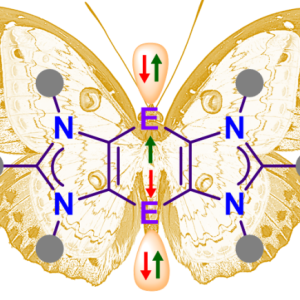
Stable radicals, whether they have one, two, or more unpaired electrons, are at the forefront of research aiming to address critical challenges in energy storage, quantum computing, and redox flow batteries. By precisely controlling their electronic and spin properties, these materials offer exciting possibilities for the next generation of functional materials. Fine-tuning their properties and integrating them into large-scale devices will be key to their success in commercial applications, driving innovation in both sustainable energy technologies and quantum computing.
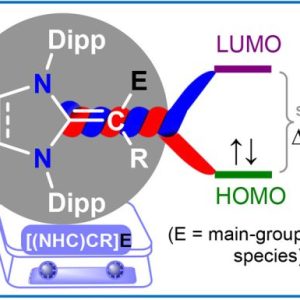
π-Conjugation is at the heart of many advanced organic functional materials, playing a crucial role in areas such as organic electronics, energy storage, sensors, and molecular magnets. By understanding and exploiting the electronic properties of conjugated systems, one can design novel materials with tailored properties for a wide range of applications. Continued advancements in the synthesis, processing, and integration of these materials will be key to unlocking their full potential in emerging technologies.

The organometallic chemistry of base metals such as iron, cobalt, nickel, and copper is at the forefront of developing sustainable catalytic systems that can replace the heavy, expensive, and toxic platinum group metals. These base metals are abundant, affordable, and environmentally friendly, offering exciting possibilities for catalysis in a wide range of applications, including green chemistry, renewable energy, and fine chemicals production. By improving catalyst performance through ligand design, reaction optimization, and hybrid systems, base metal catalysis holds the potential to transform industrial catalysis and contribute to a more sustainable future.

The development of innovative ligand systems is a cornerstone of modern molecular sciences and catalysis. By manipulating the electronic properties, structure, and coordination of ligands, chemists can enhance the selectivity, efficiency, and sustainability of catalytic reactions. Ligand design is not only pivotal in traditional areas of catalysis but also in emerging fields like green chemistry, energy conversion, and materials science. As we move forward, the continued innovation in ligand chemistry will enable breakthroughs in a wide range of applications, from clean energy technologies to advanced molecular electronics and quantum computing.

The activation of small molecules like H₂ (hydrogen), CO (carbon monoxide), CO₂ (carbon dioxide), N₂ (nitrogen), and NH₃ (ammonia) plays a crucial role in the development of sustainable synthesis, energy solutions, and green resources. These molecules are fundamental to many important chemical processes, and their activation can lead to cleaner, more efficient technologies for energy production, carbon management, and the synthesis of valuable chemicals.

© 2024 Ghadwal Research Group
Anorganische Chemie und Strukturchemie (ACS), Fakultät für Chemie
Universität Bielefeld, E4-124, Universiätsstr. 25, D-33615 Bielefeld
Tel: 0049-521-106-6167 (Off.); Fax:0049-521-106-6026
E-mail: rghadwal@uni-bielefeld.de
