Molecular Inorganic Chemistry and Catalysis
advancing low-valent main-group chemistry for sustainable synthesis, catalysis, and materials

Annulated Radical Cations with a C4E2-Core (E = P, As, Sb): Stable Pnictogen Analogs of Elusive Aryl Radical Anions of Birch Reduction Reactions
H. Steffenfauseweh, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler, D. D. Snabilié, B. de Bruin,* R. S. Ghadwal*

One-electron (1e–) oxidations of the base-stabilized cyclic bis-pnictinidene compounds 1-E afford the 7π-electron C4E2-ring-containing radical cations [2-E]●+, which are valence isoelectronic to the benzene radical anion [C6H6]●– of the Birch reduction reactions. Further 1e-oxidations of [2-E]●+ give the dications [3-E]2+ while reactions with TEMPO (or Ph2Se2) yield the monocations [4-E]+ (R = TEMPO) (or [5-P]+, R = SePh).

Lithium Anionic Dicarbenes or Acetylides: What is in the Name?
A. Merschel, H. Steffenfauseweh, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler, R. S. Ghadwal*
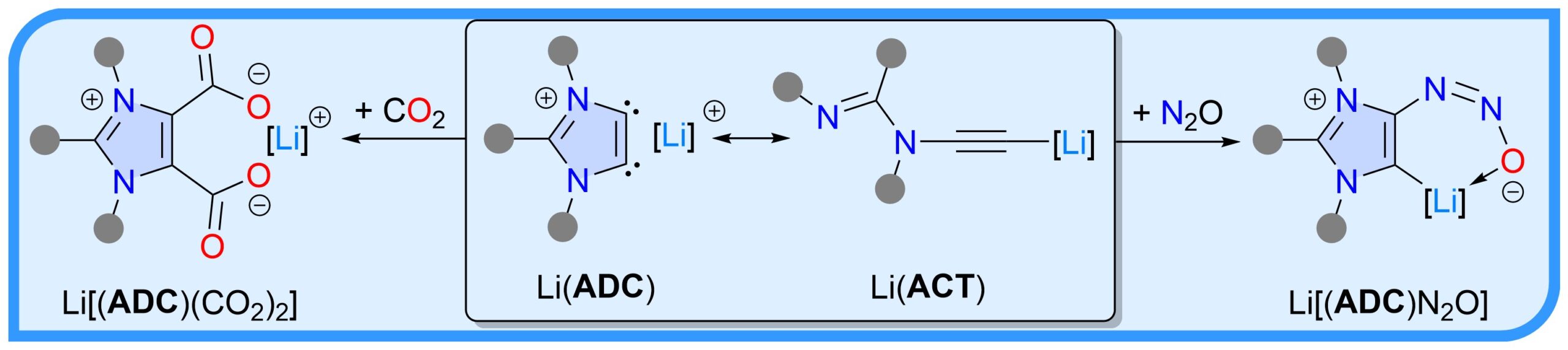
Two-fold deprotonation of the C2-arylated 1,3-imidazolium salts with nBuLi affords the so-called lithium anionic dicarbenes Li(ADC). X-Ray diffraction studies reveal that Li(ADC) exist as lithium acetylides Li(ACT). Exposure of Li(ACT) to CO2 and N2O readily afford ADC-compounds Li[(ADC)(CO2)2] and Li[(ADC)N2O], respectively.

Structural Snapshots of Reversible Carbon Dioxide Capture and (De)oxygenation at Group 14 Diradicaloids
F. Ebeler, B. Neumann, H.-G. Stammler, I. Fernández, R. S. Ghadwal*

A thorough study on CO2 activation, reversible capture, and (de)oxygenation mediated by stable Group 14 singlet diradicals (i.e. diradicaloids) [(ADC)E]2 (E = Si, Ge, Sn) based on an anionic dicarbene (ADC) framework (ADC = PhC{(N(Dipp)C}2; Dipp = 2,6-iPr2C6H3) is showcased.

Annulated 1,4-Disilabenzene-1,4-diide and Dihydrogen Splitting
F. Ebeler, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler, D. W. Szczepanik, R. S. Ghadwal*
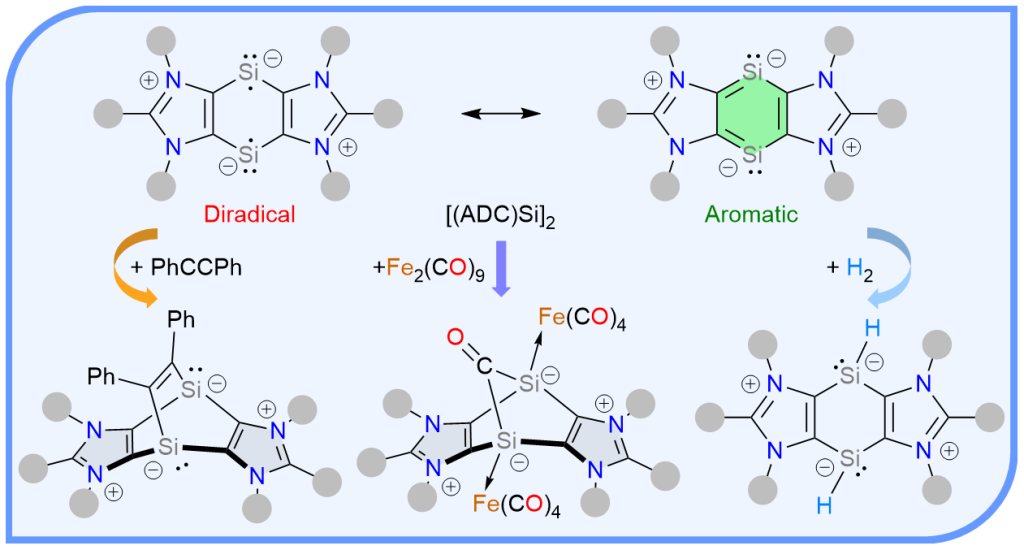
The first annulated 1,4-disilabenzene-1,4-diide compound [(ADC)Si]2 (5) based on anionic dicarbene (ADC) scaffolds (ADC = PhC{N(Dipp)C}2; Dipp = 2,6-iPr2C6H3) has been reported as a green-yellow crystalline solid. In addition to the detailed structural analyses by experimental and theoretical methods, reactivity studies of [(ADC)Si]2 have been showcased with various substrates.

Annulated Carbocyclic Gallylene and Bis-Gallylene with Two-Coordinated Ga(I) Atoms
A. Merschel, S. Heda, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler, R. S. Ghadwal*
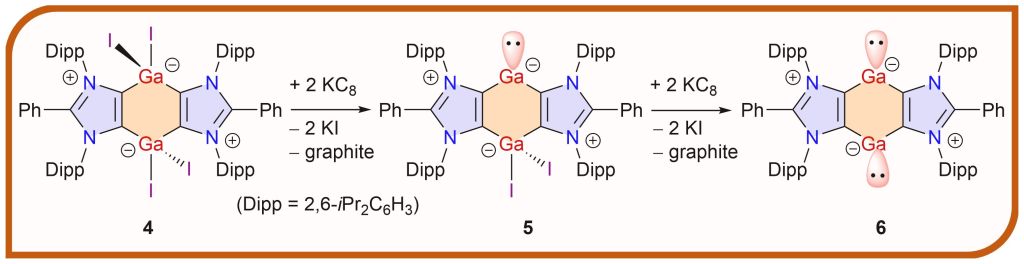
The first carbocyclic gallylene [(ADC)2Ga(GaI2)] and bis-gallylene [(ADC)Ga]2 (ADC = PhC{N(Dipp)C}2; Dipp = 2,6-iPr2C6H3) featuring a central C4Ga2 ring annulated between two 1,3-imidazole rings are prepared by KC8 reductions of [(ADC)GaI2]2.

Boosting the π-Acceptor Property of Mesoionic Carbenes by Carbonylation with Carbon Monoxide
A. Merschel, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler, R. S. Ghadwal*

Direct room temperature dimerization of carbon monoxide by anionic dicarbenes Li(ADC) has been reported to quantitatively yield (E)-ethene-1,2-bis(olate) bridged mesoionic carbene (iMIC) lithium compounds, COen-[(iMIC)Li]2. They undergo 2e-oxidation to afford 1,2-dione bridged bis iMIC, COon-(iMIC)2 compounds while redox neutral salt metatheses yield COen-[(iMIC)E]2 containing compounds (E = main group element).

Isolation of an Anionic Dicarbene Embedded Sn2P2 Cluster and Reversible CO2 Uptake
F. Ebeler, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler, R. S. Ghadwal*
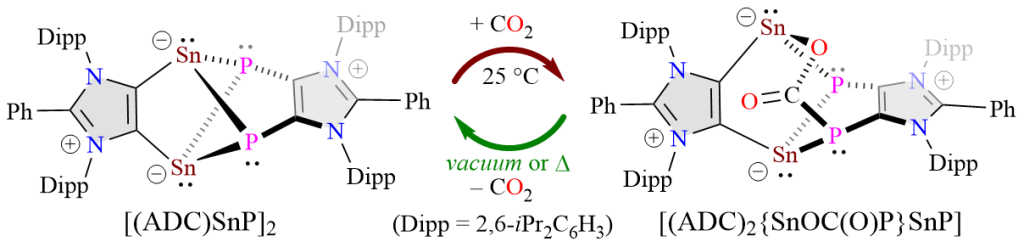
The Sn2P2 cluster compound [(ADC)SnP]2 based on an anionic dicarbene (ADC) has been isolated as a green crystalline solid. [(ADC)SnP]2 reversibly binds with CO2 at room temperature to form [(ADC)2{SnOC(O)P}SnP] and enables catalytic hydroboration of CO2.

1,3-Imidazole Based Mesoionic Carbenes and Anionic Dicarbenes: Pushing the Limit of Classical N-Heterocyclic Carbenes
R. S. Ghadwal*

MiniReview: Mesoionic carbenes (iMICs) and anionic dicarbenes (ADCs) are readily accessible by the deprotonation of C2-arylated 1,3-imidazolium salts, which are prepared by the direct C2-arylation of classical N-heterocyclic carbenes (NHCs). The implications of iMICs as potent σ-donor ligands for organometallic catalysis and ADCs as unique building-blocks to access conceptually new main-group heterocycles with an annulated C4E2 ring have been showcased.

Crystalline Anions Based on Classical N-Heterocyclic Carbenes
A. Merschel, D. Rottschäfer, B. Neumann, H.-G. Stammler, M. Ringenberg, M. van Gastel, T. I. Demirer, D. M. Andrada., R. S. Ghadwal*
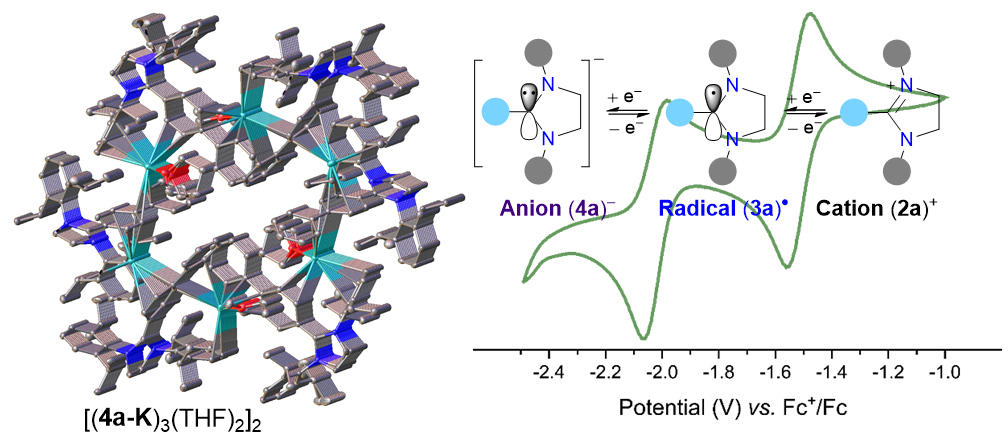
One-electron reduction of the C2-biphenylated 1,3-imidazolinium cation (2a+) affords the radical 3a, which undergoes further 1e-reduction to yield the anion 4a–. In the solid-state, 3a is monomeric while 4a-K has a hexameric tubular structure. In addition to 3a and 4a-K, synthesis, structures, and reactivity of stable radicals and anions based on unsaturated N-heterocyclic carbene frameworks have been presented.

Isolation of an Annulated 1,4-Distibabenzene Diradicaloid
H. Steffenfauseweh, D. Rottschäfer. Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler, D. W. Szczepanik, R. S. Ghadwal*
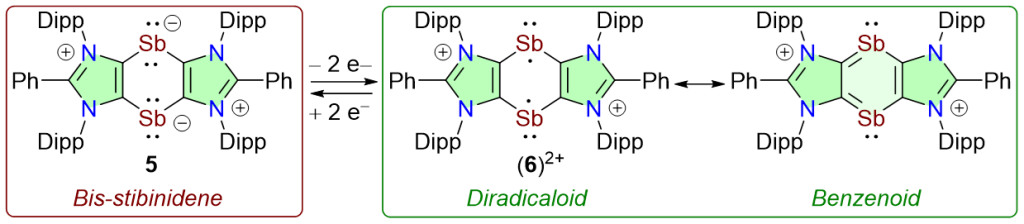
The first 1,4-distiba-1,4-diide (i.e. cyclic bis-stibinidene) 5 is isolated as a crystalline solid. 5 undergoes 2e-oxidation to yield the dicationic compound (6)2+, which can be reduced back to 5. The broken-symmetry open-shell singlet solution for (6)2+ is calculated to be 2.13 kcal/mol more stable than the closed-shell singlet solution. (6)+2 is stabilized by the delocalization of unpaired electrons over the benzenoid C4Sb2 ring and has 39% diradical character (y).

Isolation of an Arsenic Diradicaloid with a Cyclic C2As2-Core
H. Steffenfauseweh, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler, D. M. Andrada, R. S. Ghadwal*
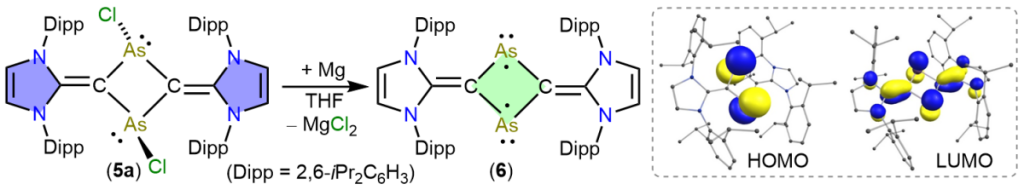
The C2As2-cyclic diradicaloid 6 is isolated as a red crystalline solid via 2e-reduction of 5a. Calculations suggest a singlet ground state for 6, which is 0.34 kcal/mol higher than the broken-symmetry open-shell singlet solution. The HOMO of 6 is located at the arsenic atoms and is trans-annular antibonding, while the LUMO is trans-annular bonding and spans over the C2As2C2-framework. Reactivity studies of 6 are shown with (PhSe)2 and Fe2(CO)9.

Tuning the Electronic Properties of Main-Group Species by N-Heterocyclic Vinyl (NHV) Scaffolds
R. S. Ghadwal*
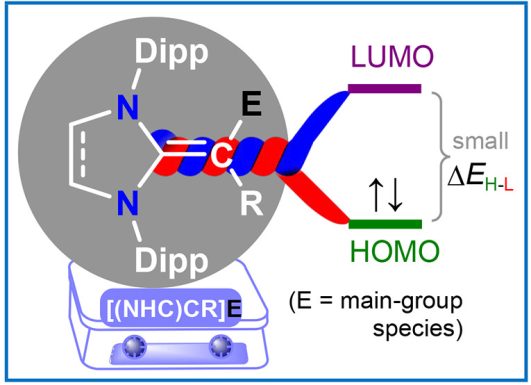
Molecules and materials with easily tunable electronic structures and properties are at the forefront of contemporary research. π-Conjugation is fundamental in organic chemistry and plays a key role in the design of molecular materials. In this Account, we showcase the applicability of N-heterocyclic vinyl (NHV) substituents based on classical N-heterocyclic carbenes (NHCs) for tuning the structure, properties, and stability of main-group species (E) via π-conjugation and/or π-donation…

Isolation of 1,4-Diarsinine-1,4-diide and 1,4-Diarsinine Derivatives
D. Rottschäfer, T. Glodde, B. Neumann, H.-G. Stammler, D. M. Andrada, R. S. Ghadwal*
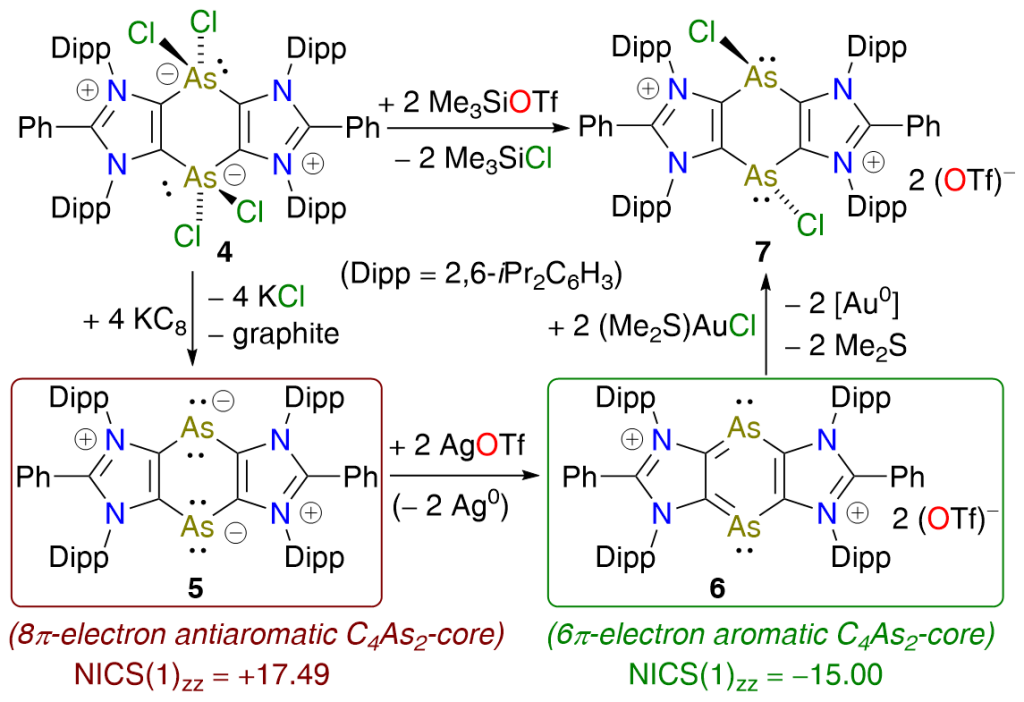
KC8 reduction of a hypervalent arsenic heterocycle 4 affords the 1,4-diarsinine-1,4-diide 5 as a red crystalline solid. The C4As2-ring of 5, formally comprising 8π-electrons, is planar and exhibits antiaromatic character. Two electron oxidation of 5 with AgOTf gives the 6π-electron C4As2-compound 6, which is an aromatic system.

An Open-Shell Singlet Sn(I) Diradical and H2 Splitting
M. Sharma, D. Rottschäfer, T. Glodde, B. Neumann, H.-G. Stammler, R. S. Ghadwal*
Angew. Chem. Int. Ed. 2021, 60, 6414–6418. Angew. Chem. 2021, 133, 6485–6489.
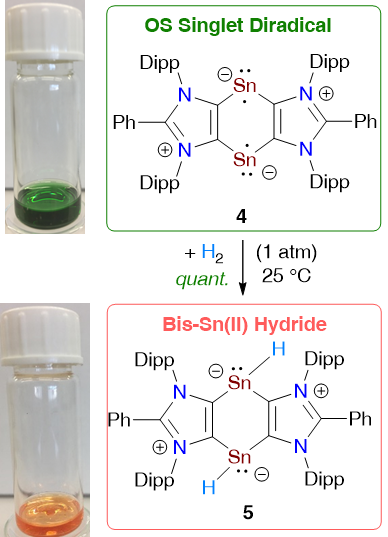
A 1,4-distannabenzene derivative 4 with two-coordinated Sn(I) atoms has been isolated as a green crystalline solid. The ground state of 4 is an open-shell singlet (OS) with a singlet-triplet energy gap (ΔEOS-T) of 4.4 kcal/mol (according to CASSCF, ΔES-T = 6.6 kcal/mol). Consequently, 4 exhibits a half-field EPR signal at 100 K and undergoes H2 splitting at room temperature to quantitatively yield the Sn(II) hydride 5 as an orange solid.

Isolation of a Ge(I) Diradicaloid and Dihydrogen Splitting
M. K. Sharma, F. Ebeler, T. Glodde, B. Neumann, H.-G. Stammler, R. S. Ghadwal*
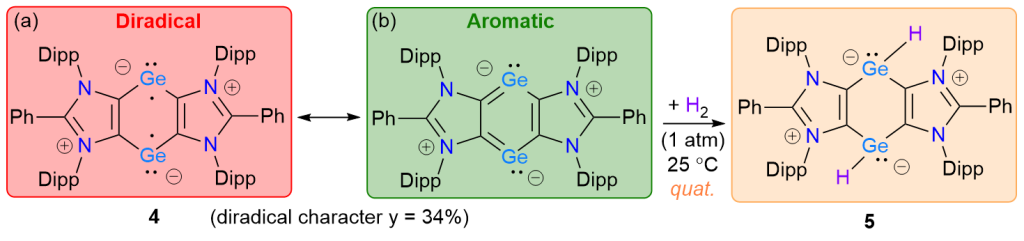
The cyclic Ge(I) compound [(ADCPh)Ge]2 (4) (ADCPh = {CN(Dipp)}2CPh, Dipp = 2,6-iPr2C6H3) containing a 6π-electron C4Ge2 framework has been isolated as a red crystalline solid. CASSCF calculations reveal a closed-shell singlet ground state for 4 with a considerable diradical character (y = 34%). Thus, the diradicaloid 4 readily splits dihydrogen at room temperature to yield the elusive bis-hydridogermylene [(ADCPh)GeH]2 (5).

Nickel Catalyzed Intramolecular 1,2‐Aryl Migration of Mesoionic Carbenes (iMICs)
A. Merschel, T. Glodde, B. Neumann, H.-G. Stammler, R. S. Ghadwal*

Super-iMICs (5) with the largest buried volume (Vbur. = 45%) known thus far for iMICs are accessible via unprecedented Ni-catalyzed intramolecular 1,2-aryl migration of C5-protonated iMICs (2) to 3, subsequent N-alkylation of 3 to 4, and the deprotonation of 4. S-iMICs (5) are stronger σ-donors and superior π-acceptors than 2. A comparative catalytic study reveals the superior activity of S-iMICs (5) over iMICs (2) and NHC (IPr).

A Crystalline C5-Protonated 1,3-Imidazol-4-ylidene
D. Rottschäfer, T. Glodde, B. Neumann, H.-G. Stammler, R. S. Ghadwal*

The first C5-protonated 1,3-imidazole-based mesoionic carbene (iMICBp) has been isolated and characterized by single-crystal X-ray diffraction.

Isolation of Singlet Carbenes Derived 2-Phospha-1,3-butadienes and their Sequential One-electron Oxidation to Radical Cations and Dications
M. K. Sharma, S. Blomeyer, T. Glodde, B. Neumann, H.-G. Stammler, M. van Gastel, A. Hinz, R. S. Ghadwal*
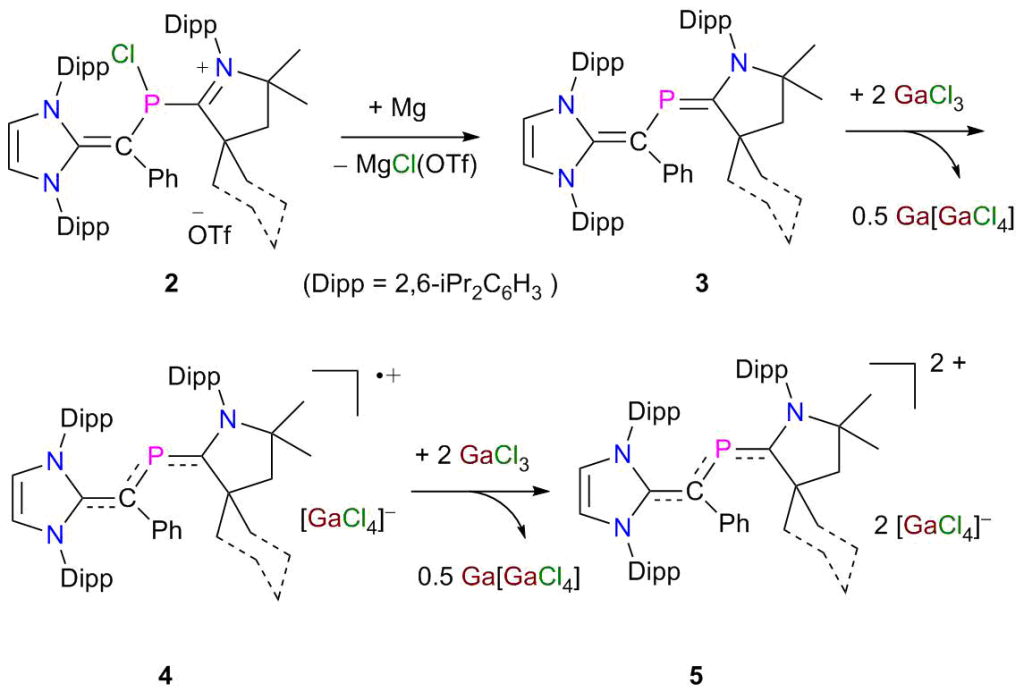
Phosphorus π-Radicals: The 2-phospha-1,3-butadienes derivatives 3 are readily accessible on reduction of 2 with Mg. Sequential one-electron oxidation of 3 with GaCl3 leads to the formation of radical cations 4 and dications 5 as crystalline solids. Molecular structures of 3–5 have been established by X-ray diffraction and analyzed by DFT calculations.

Crystalline Divinyldiarsene Radical Cations and Dications
M. K. Sharma, S. Blomeyer, B. Neumann, H.-G. Stammler, M. van Gastel, A. Hinz, R. S. Ghadwal*
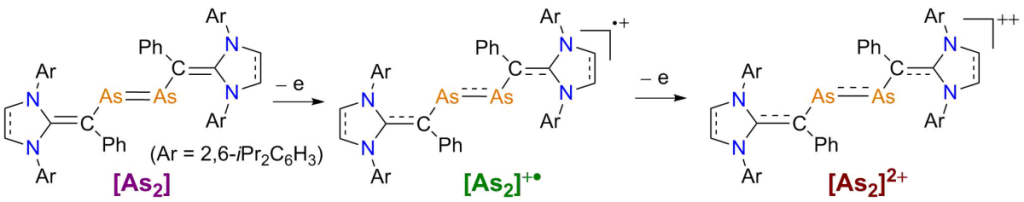
One-by-one electron oxidation of diarsenes [As2] featuring very efficient π-donor N-heterocyclic vinyl substituents with GaCl3 leads to the formation of radical cations [As2]+• and dications [As]++ as crystalline solids. Experimental and computational studies revealed delocalization of the unpaired electron over the π-conjugated CAs2C-framework

Direct Functionalization of White Phosphorus with Anionic Dicarbenes and Mesoionic Carbenes: Facile Access to 1,2,3-Triphosphol-2-ides
D. Rottschäfer, S. Blomeyer, B. Neumann, H.-G. Stammler, R. S. Ghadwal*

Unprecedented [3+1] fragmentation of white phosphorus (P4) and thus the capturing of the P3+ fragment with anionic dicarbenes (ADCs) has been shown to afford the 1,2,3-triphosphol-2-ides I in 93-98% yield. The mesoionic heterocycles I feature 6π-electron C2P3 and C3N2 aromatic systems and serve as two-electron σ-donor ligands.

N-Heterocyclic Carbene Analogues of Thiele and Chichibabin Hydrocarbons
D. Rottschäfer, N. K. T. Ho, B. Neumann, H.-G. Stammler, M. van Gastel, D. M. Andrada, R. S. Ghadwal*

Stable NHC-analogues of Thiele’s and Chichibabin’s hydrocarbons [(IPr)(C6H4)(IPr)] (4) and [(IPr)(C6H4)2(IPr)] (5) (IPr = C{N(2,6-iPr2C6H3)}2CHCH) are reported.

Crystalline Radicals Derived from Classical N-Heterocyclic Carbenes
D. Rottschäfer, B. Neumann, H.-G. Stammler, M. van Gastel, D. M. Andrada, R. S. Ghadwal*
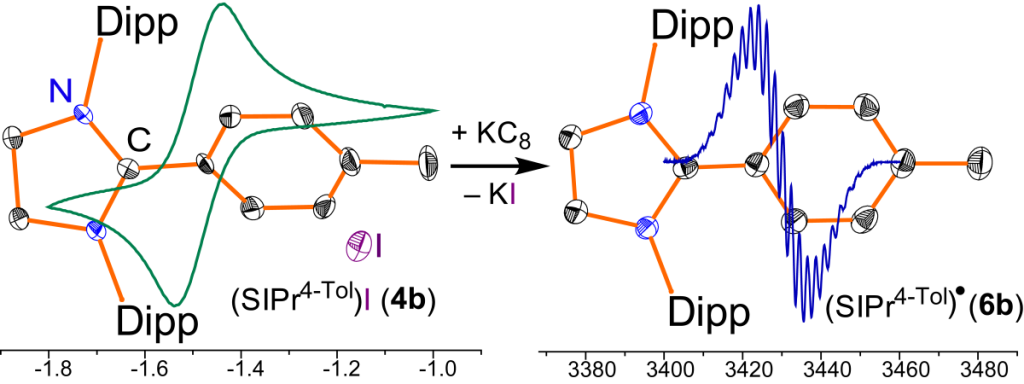
Crystalline radicals (IPrAr)• (5) and (SIPrAr)• (6) derived from classical N-heterocyclic carbenes (NHCs), (IPr = :C{N(2,6-iPr2C6H3)}2CHCH and SIPr = :C{N(2,6- iPr2C6H3)}2CH2CH2) are readily accessible by one electron reduction of the corresponding C2-arylated 1,3-imidazoli(ni)um cations 3 and 4. Cyclic voltammetry, EPR, and X-ray diffraction studies as well as DFT calculations emphasize the key role of C2-substituent in the stability of NHC-derived radicals.

© 2024 Ghadwal Research Group
Anorganische Chemie und Strukturchemie (ACS), Fakultät für Chemie
Universität Bielefeld, E4-124, Universiätsstr. 25, D-33615 Bielefeld
Tel: 0049-521-106-6167 (Off.); Fax:0049-521-106-6026
E-mail: rghadwal@uni-bielefeld.de
